Get to know the areas where Biocen do Brasil acts.
Biocen do Brasil’s CromoCen product line efficiently monitors and guarantees the microbiological quality in the pharmaceutical sector.
The certification of microbiological quality in the cosmetic industry is asserted by Biocen do Brasil’s CromoCen product line.
Biocen do Brasil supplies its CromoCen products to the agro-food industry. The product line ensures precision, efficiency, and reliability in the microbiological analyses.
Click here and get to know the CromoCen product line for the water industry.
CERTIFICATES
Download your certificate.
Biocen do Brasil is a company that produces only ready-to-use culture media.
Since its foundation, Biocen do Brasil works to ensure services of the highest quality, envisioning the society’s well being. The commitment of microbiology professional scientists reflects the rising development of innovative products supplied at a national level. Learn more..
Get to know our products
Learn more about our available products.
Microbiology Solutions
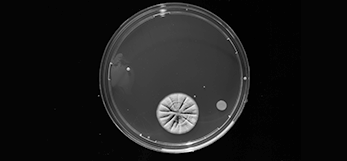
Environmental monitoring
Environmental monitoring in classified areas is essential for ensuring the quality of microbiology products. It is crucial for following sanitary standards and regulations, understanding the microbial flora, developing trend analyses, and controlling the processes of production.

Water Analysis
Microbiological monitoring of water systems is essential to ensure the quality of production, storage, and distribution of culture media plates. Meeting sanitary standards and regulations, the process undergoes a thorough analysis for its installation and validation.
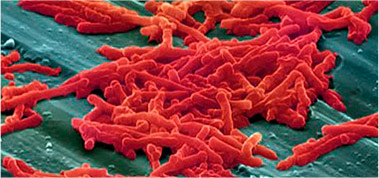
Analysis of Pathogens
Our high quality culture media efficiently identifies pathogenic microorganisms according to the ruling pharmacopoeia.

Media Fill

Sterility Analysis
Products categorized as steril must pass through a series of sterility tests, in which Fluid Thioglycollate and Tryptic Soy Broth culture media are used to ensure that there are no contaminations in the samples.
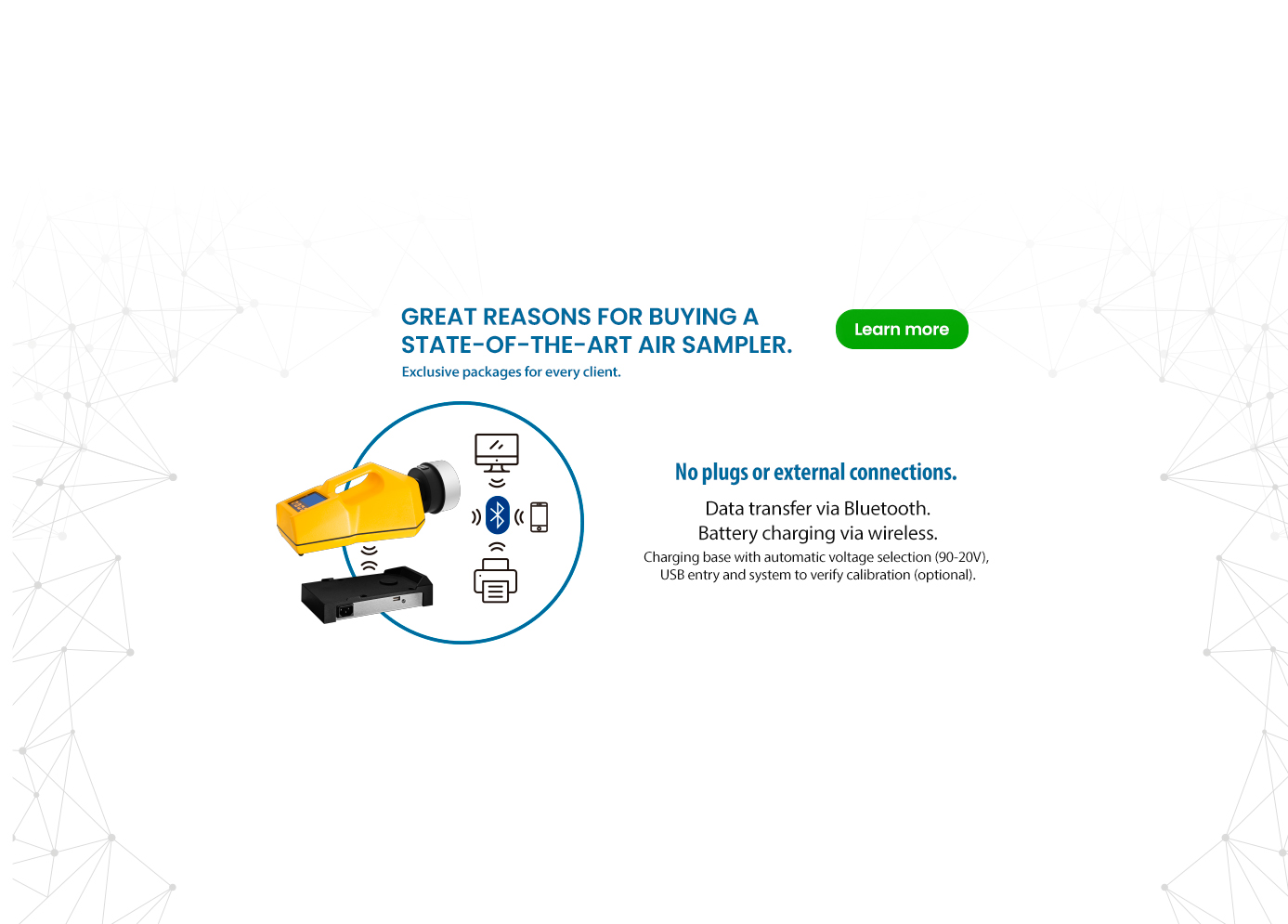
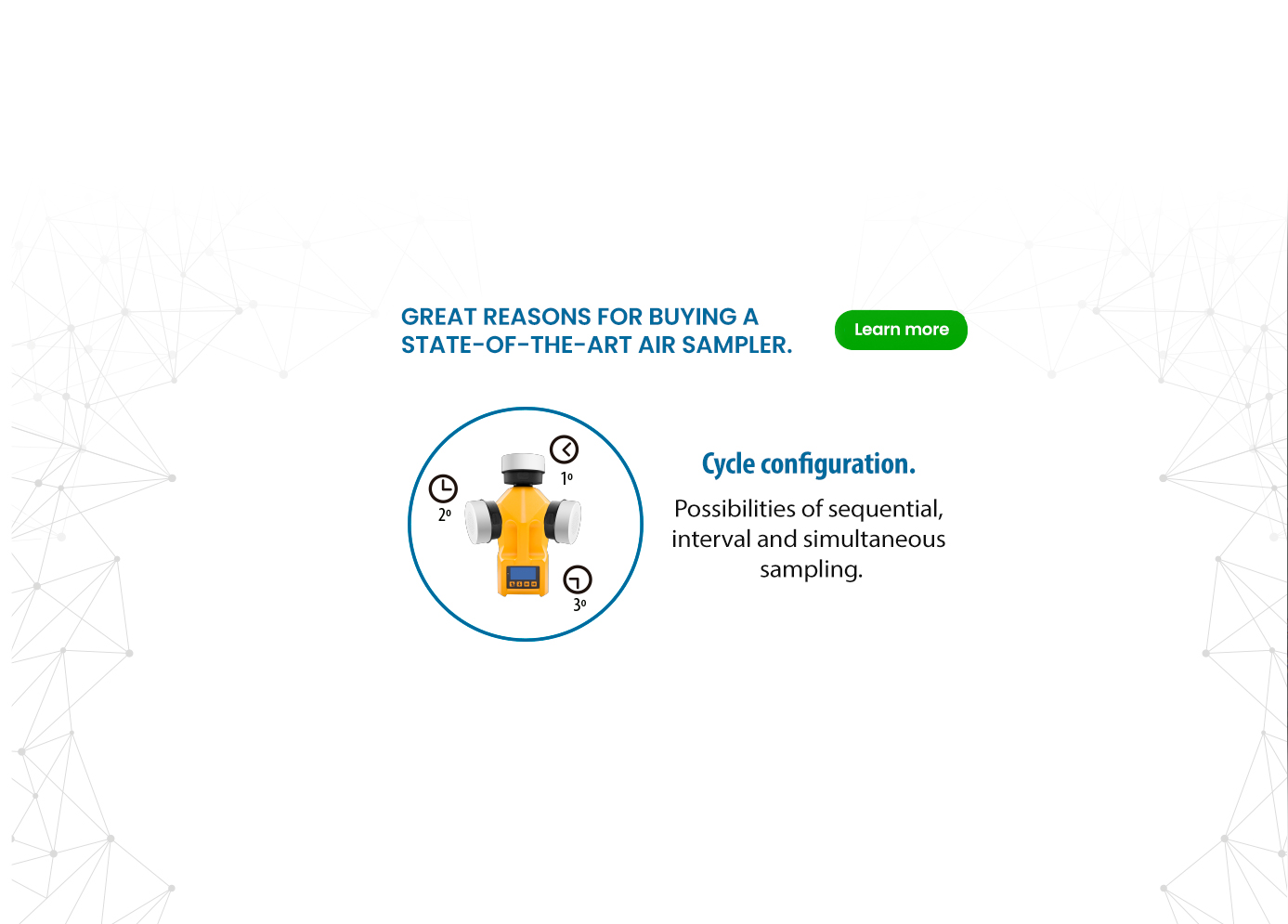
Why Biocen do Brasil?
1 – Biocen do Brasil produces only ready-to-use culture media that are irradiated by gamma rays within 24 hours of its production.
2- It focuses on providing high quality specialized services for its clients.
Differential
Punctual deliveries, customized products, high quality raw materials and pharmaceutical scientist professionals.

News
FEW MICROBIOLOGICAL NOTES ABOUT AIR SAMPLING IN CLEANROOM
MICROBIAL AIR SAMPLER POSITIONING IN CLEANROOM / ISOLATOR / RABS
MONITORING IN CLEANROOM
![]()